RESPOND-CRT is an International, multicenter, randomized (2:1), prospective, non-inferiority study.1
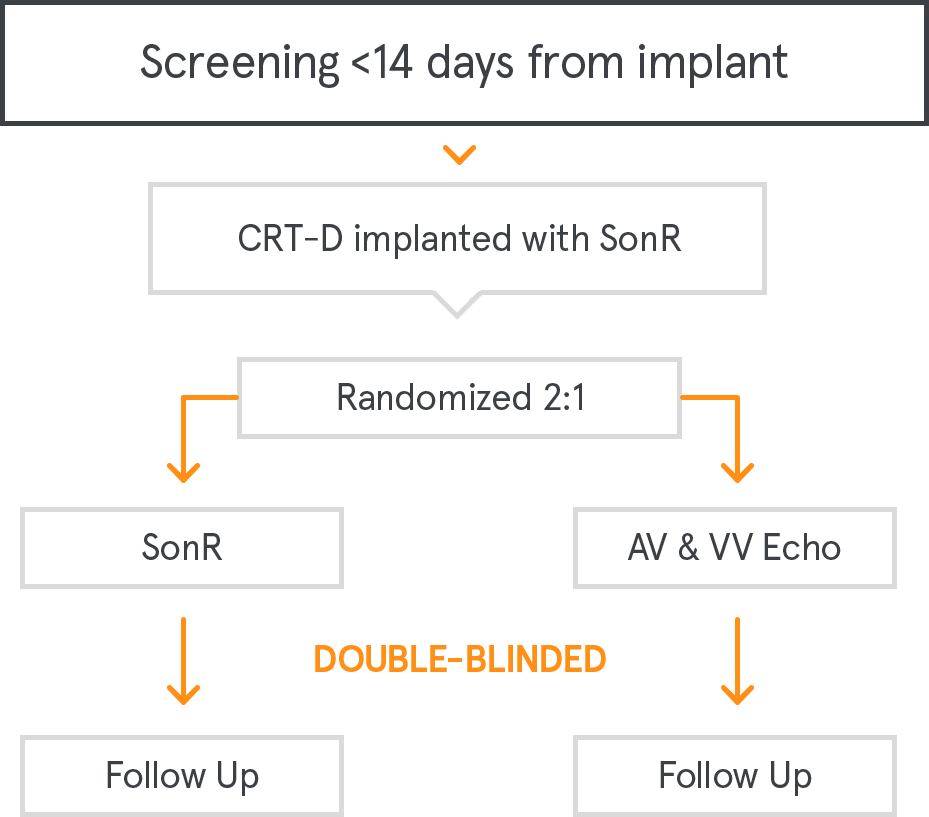
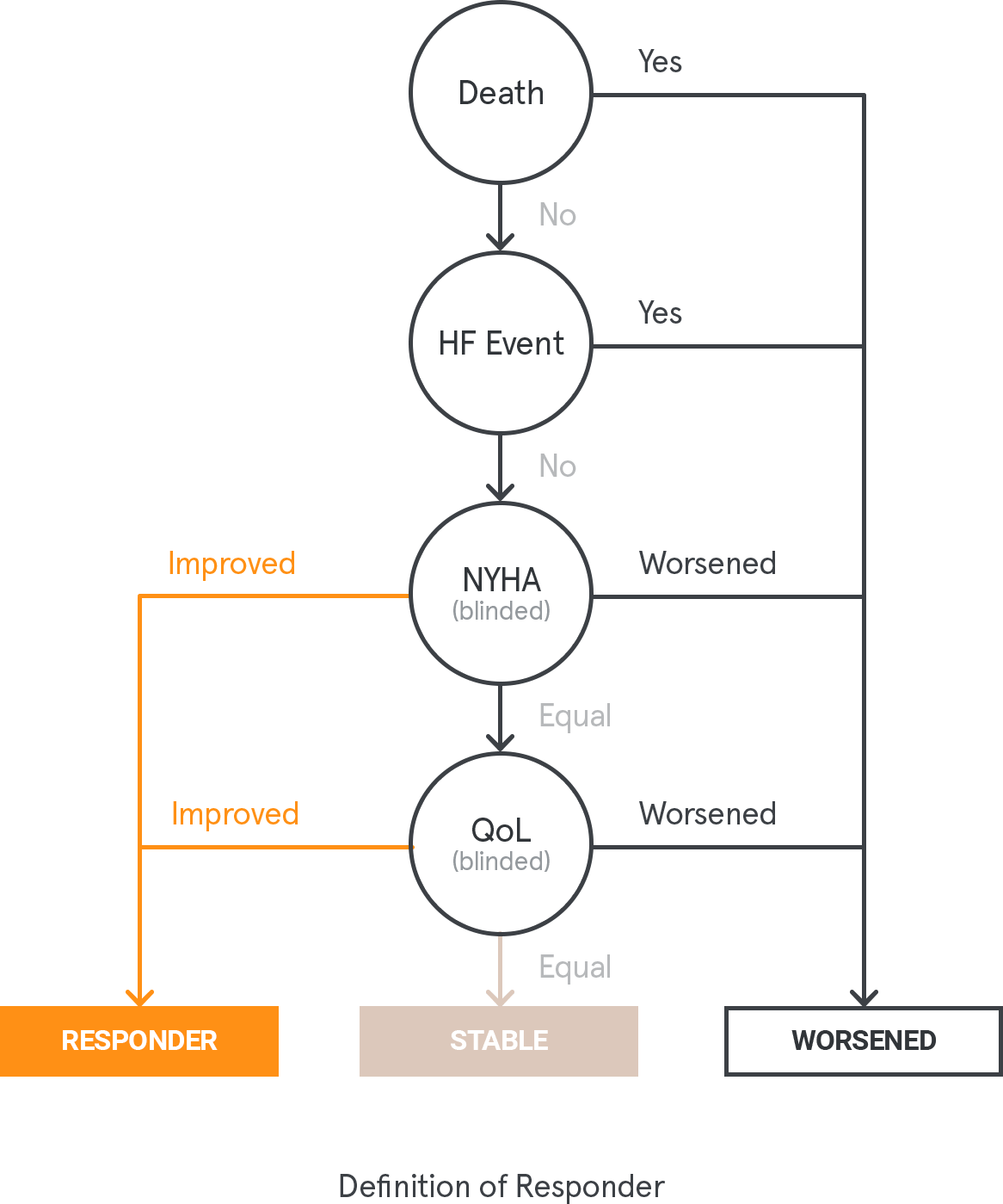
Non-inferiority on the proportion of responders, based on a clinical composite of criteria (10% non-inferiority margin), at 12 months
Freedom from acute (0-3 months) and chronic (3-12 months) SonRtip lead complications
1. Brugada J, Brachmann J, Delnoy PP et al. Automatic optimization of cardiac resynchronization therapy using SonR-rationale and design of the Clinical trial of the SonRtip lead and Automatic AV-VV optimization Algorithm in the Paradym RF SonR CRT-D (RESPOND CRT) trial. Am Heart J , 2014 ; Vol 167 (4):429-435

Where do we stand after two decades of trying to find the right CRT optimization strategy? Experts discuss the results of the RESPOND-CRT trial and the implications for clinical practice.

SonR technology is available exclusively in LivaNova devices. Learn more about the Platinium SonR™ family of CRT-D devices.
This site is intended for healthcare professionals outside the United States of America. By continuing to use this site you are confirming that that you are a healthcare professional based outside the USA.